AD Dr. Davide Ciliberti: Cognitive Electrophysiology and Neurotechnology
Description of the research group
The Cognitive Electrophysiology and Neurotechnology (CEN) group has two interlinked goals:
- to understand how the formation, maintenance and retrieval of human memory is supported by the electrophysiological activity of the brain;
- to develop neurotechnologies which can help us understand the cognitive role of human electrophysiological activity patterns and treat neurological disorders such as memory loss.
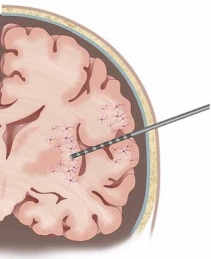
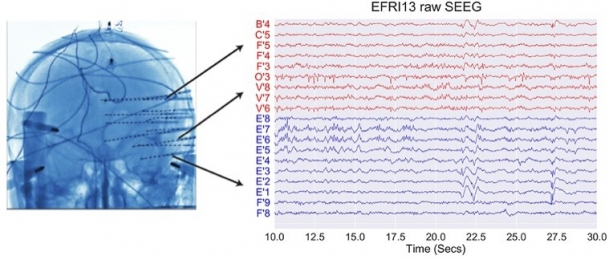
Our research leverages on the rare opportunity of measuring brain activity intracranially in patients affected by pharmaco-resistant epilepsy. In pharmaco-resistant epilepsy patients, neurologists use intracranial electroencephalography (iEEG) to delineate the epileptogenic focus, when non-invasive scalp EEG and other techniques have been insufficient to localize it. iEEG is recorded with intracranial electrodes and it has higher spatial resolution and higher signal-to-noise ratio than scalp EEG.
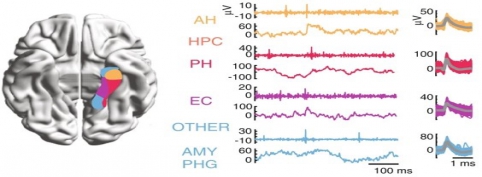
iEEG is generally more used in mesial temporal lobe epilepsy. Therefore, the clinical intracranial macroelectrodes often record electrophysiology signals from the hippocampus, the entorhinal cortex and the amygdala, which are very important for cognitive processes such as memory. However, other areas, such as the frontal cortex, are also not uncommon implantation targets.
 |
 |
| image credits [1] | image credits [2] |
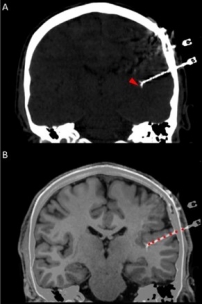
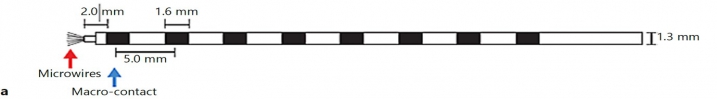
In addition to the very valuable iEEG recordings from the clinical macroelectrodes, our iEEG recordings also include signals recorded with microelectrodes. The additional microelectrodes have a diameter of about 40 μm (smaller than the hair) and spread out of the tip of the clinical intracranial electrode. They are added solely for research purposes and, because of their small size, they can record the electrophysiological firing activity generated by nearby individual neurons. We analyze this data by using algorithms which classify the different waveforms recorded by a single microwire into action potentials generated by different neurons. We also use population decoding methods to extract the information associated by the neurons (e.g. “concept” neurons which preferably fire in response to specific images of the same individual). To localize the site of the macro- and micro-electrodes with high spatial resolution, we combine post-implantation CT scans with pre-implantation neuroimaging scans from the 7-Tesla MRI, available at the University of Magdeburg.
 |
 |
|
| image credits [4] | ||
 |
||
| image credits [3] | image credits [5] | |
With intracranial electrodes, we can not only record the brain’s electrophysiological activity at neuronlevel spatial resolution, but we can also modulate this activity via electrical deep-brain stimulation (DBS). Because we combine iEEG recordings and DBS with cognitive tasks (e.g. mnemonic discrimination tasks), we can infer links between brain activity and human memory. Electrical stimulation is delivered through either the macro- or microelectrodes in specific temporal patterns, mimicking physiological activation.
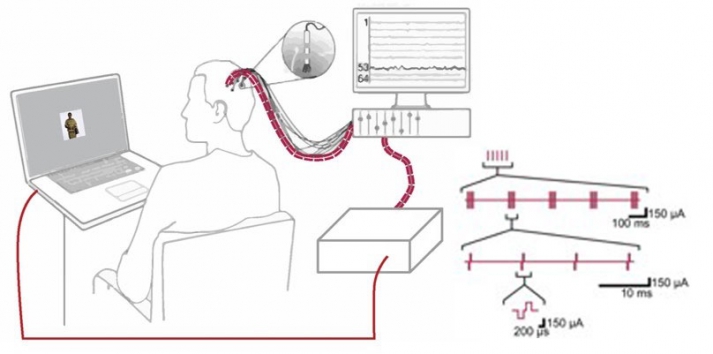 |
| image credits [6] |
In order to pinpoint cause and effect between specific electrophysiological patterns and cognition, we deploy closed-loop technologies. During closed-loop experiments, a real-time hardware-software system ensures that DBS is precisely timed to the occurrence of specific short-lasting (~40-1000 ms) electrophysiological patterns, such as fast oscillations (~80-120 Hz), bursts of firing neurons or phases of an underlying rhythm (e.g. theta at ~4-8 Hz). For this purpose, we take advantage of Falcon, a software platform for closed-loop experiments, which can deliver a stimulation to brain with submillisecond round-trip latency and can adapt to a variety of experiments.
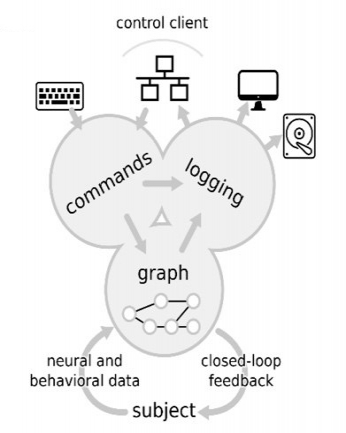 |
| image credits [7] |
Last but not least, we are also interested in improving the current microelectrode technology for studying cognition in humans. Our goal is to upscale the number of simultaneously recorded neurons.
 |
| image credits [8] |
In the long run, we aim to lay the grounds for the development of a cognitive neuroprosthesis: a brain implant which can restore the ability to store and retrieve information in people suffering from memory disorders.
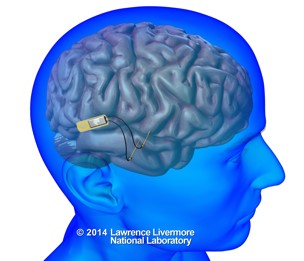 |
| image credits [9] |
Research streams
- Circuit-specific manipulations of the hippocampo-entorhinal subnetworks via DBS (CRC project).
- Study of the effect of non-invasive stimulation (electrical and focused ultra-sound) on the activity of individual neurons (CRC project).
- Closed-loop manipulation of hippocampal transient patterns during sleep-mediated memory consolidation.
- Adaptive methods for the functional screening of single-neuron activity.
- Study of pattern separation at the level of single-neuron activity.
- Closed-loop enhancement of memory encoding/decoding via microstimulation.
- Upscaling of human single-unit microwire recording technology.
Clinical partners
- Dr. med. Friedhelm C. Schmitt (OvGU, Head of Epileptology, Department of Neurology)
- Prof. Dr. med. Erol Sandalcioglu (OvGU, Director of Neurosurgery)
Collaborators
The CEN lab is part of the CRC1436 collaborative research group
(https://sfb1436.de/members/#member-21949-info). Collaborators include:
- Prof. Rodrigo Q. Quiroga (visiting Professor at OvGU, ICREA Research Professor, Germany/Spain)
- Prof. Dr. med. Dr. rer. nat. Florian Mormann (University of Bonn, Germany)
- Prof. Dr. Magdalena Sauvage (Leibniz Institute for Neurobiology, Germany)
- Prof. Dr. Frank Angenstein (DZNE, Germany)
- Dr. rer. nat. Stefan Dürschmid (Leibniz Institute for Neurobiology, Germany)
- Prof. Dr. Thomas Wolbers (DZNE, Germany)
- Prof. Jonathan Kao (UCLA, USA)
- Dr. Emily Mankin (UCLA, USA)
- Prof. Marc Van Hulle (KU Leuven, Belgium)
- Prof. Luciano Fadiga (University of Ferrara, Italy)
Funding
The group is supported by the EFRE project “Electrophysiology of human memory: invasive brain recording and brain stimulation”.
Open positions
The lab is currently recruiting a research assistant, a PhD student and a postdoc. Please get in touch if you are interested in joining the group.
Image credits
1. © Grande et al. 2020. The image is a cropped version of the original. Reproduced according to the terms of Creative Commons Attribution License. Grande, Krista M., Sarah K. Z. Ihnen, and Ravindra Arya. 2020. “Electrical Stimulation Mapping of Brain Function: A Comparison of Subdural Electrodes and Stereo-EEG.” Frontiers in Human Neuroscience 14 (December). https://doi.org/10.3389/fnhum.2020.611291.
2. © Greene et al., 2021. Reproduced according to the terms of Creative Commons Attribution License. Greene, Patrick, Adam Li, Jorge González-Martínez, and Sridevi V. Sarma. 2021. “Classification of Stereo-EEG Contacts in White Matter vs. Gray Matter Using Recorded Activity.” Frontiers in Neurology 11 (January). https://doi.org/10.3389/fneur.2020.605696.
3. © Lehongre et al., 2022. Reproduced according to the terms of Creative Commons Attribution License. Lehongre, Katia, Virginie Lambrecq, Stephen Whitmarsh, Valerio Frazzini, Louis Cousyn, Daniel Soleil, Sara Fernandez-Vidal, et al. 2022. “Long-Term Deep Intracerebral Microelectrode Recordings in Patients with Drug-Resistant Epilepsy: Proposed Guidelines Based on 10-Year Experience.” NeuroImage 254 (July):119116. https://doi.org/10.1016/j.neuroimage.2022.119116.
4. © Carlson et al., 2018. Reproduced according to the terms of Creative Commons Attribution License. Carlson, April A., Ueli Rutishauser, and Adam N. Mamelak. 2018. “Safety and Utility of Hybrid Depth Electrodes for Seizure Localization and Single-Unit Neuronal Recording.” Stereotactic and Functional Neurosurgery 96 (5): 311–19. https://doi.org/10.1159/000493548.
5. © Umbach et al., 2022. Reproduced according to the terms of Creative Commons Attribution License. Umbach, Gray, Ryan Tan, Joshua Jacobs, Brad E. Pfeiffer, and Bradley Lega. 2022. “Flexibility of Functional Neuronal Assemblies Supports Human Memory.” Nature Communications 13 (1): 6162. https://doi.org/10.1038/s41467-022-33587-0.
6. © Mankin et al., 2021, © Titiz et al., 2017. The large image on the left was created by Mankin et al., 2021. Mankin, Emily A., Zahra M. Aghajan, Peter Schuette, Michelle E. Tran, Natalia Tchemodanov, Ali Titiz, Güldamla Kalender, et al. 2021. “Stimulation of the Right Entorhinal White Matter Enhances Visual Memory Encoding in Humans.” Brain Stimulation 14 (1): 131–40. https://doi.org/10.1016/j.brs.2020.11.015. The smaller image on the right was created by Titiz et al., 2017. Titiz, Ali S, Michael R H Hill, Emily A Mankin, Zahra M Aghajan, Dawn Eliashiv, Natalia Tchemodanov, Uri Maoz, et al. 2017. “Theta-Burst Microstimulation in the Human Entorhinal Area Improves Memory Specificity.” Edited by Inna Slutsky. eLife 6 (October):e29515. https://doi.org/10.7554/eLife.29515. Both images are reproduced according to the terms of Creative Commons Attribution License.
7. © Ciliberti & Kloosterman, 2017. Ciliberti, Davide, and Fabian Kloosterman. 2017. Reproduced according to the terms of Creative Commons Attribution License. “Falcon: A Highly Flexible Open-Source Software for Closed-Loop Neuroscience.” Journal of Neural Engineering 14 (4): 045004. https://doi.org/10.1088/1741-2552/aa7526.
8. © Natalie Cherry and Davide Ciliberti, 2021.
9. © Lawrence Livermore National Laboratory, 2014. Reproduced according to the terms of Creative Commons Attribution License. “DARPA Taps Lawrence Livermore to Develop World’s First Neural Device to Restore Memory.” 2014. https://www.llnl.gov/article/40181/darpa-taps-lawrence-livermore-develop-worlds-first-neural-device-restore-memory. his work is licensed under CC BY-NC-SA 4.0.







