SFB 1436
 -
-
Neural Resources of Cognition
CRC 1436 is an integrative center established recently in Magdeburg, Germany, by the Otto von Guericke University, the German Center for Neurodegenerative Diseases (DZNE) and the the Leibniz Institute for Neurobiology (LIN). It is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB-1436 - Project-ID 425899996.
The innovative research program of the CRC deals with the fundamental question of what are the neurobiological principles that constitute and limit neural resources of cognition and constrain the potential to fully utilize or to even increase these resources. Using intervention-based research projects in humans and animal models, we aim to understand individual variability, including aging and superaging, and transferrable enhancement of cognitive functions. We will consider important modifiers such as tau- and amyloid-pathology, sleep deprivation and vascular reserve. We will utilize a wide range of cutting-edge and unique imaging technologies.
PhD students will be admitted to the local graduate program of the CRC with outstanding opportunities in multidisciplinary and interconnected scientific fields supported by the mentoring and networking opportunities of the CRC 1436.
For more details on the research topics, the qualification program, please check the following links:
Governing bodies of the Collaborative Research Centre
| Steering committee | |
| Spokesperson: | Prof. Dr. med. Emrah Düzel |
| Deputy spokesperson: | Dr. rer. nat. Michael R. Kreutz |
| Executive board: |
Prof. Dr. rer. nat.Daniela C. Dieterich |
| Advisory members: | Prof. Dr. rer. nat. Eckart D. Gundelfinger Prof. Dr. med. Hans-Jochen Heinze |
| contact person: | Rödiger, Julia |
Research profile of the Collaborative Research Centre
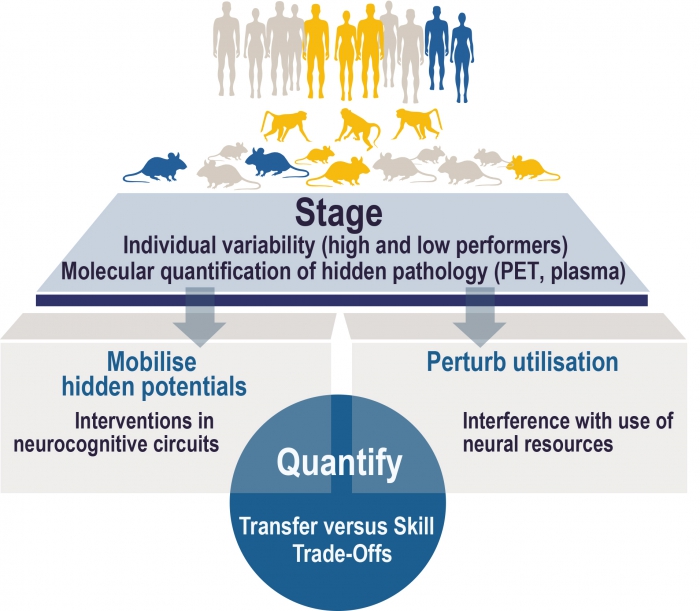
Summary of the research programme
Neuroscientists have made considerable progress in delineating neural circuits governing cognitive functions, and in understanding fundamental component mechanisms of cognitive faculties. These conceptual advances together with recent developments in imaging technology now pave the way to systematically address one of the most pressing and obvious questions in cognition research: What are the neurobiological principles that constitute and limit neural resources of cognition and constrain the potential to fully utilise or to even increase these resources? The notion that neural resources are limited and vulnerable is central to major concepts that seek to explain individual variability in cognitive performance, changes across the lifespan and in age-related cognitive decline (i.e.reserve, resilience, resistance, maintenance). Despite this central role, a neuro-biological understanding of neural resources is still lacking. Thus, a fundamental knowledge gap se-verely constrains our ability to develop overarching theories explaining individual variability in animal and human cognition and in the capability to preserve or enhance cognitive performance over the lifespan as well as in the face of pathology.
It is now very timely to embark on a systematic investigation into the neurobiology of neural resources. Powerful tools and technology have recently become available to link neurobiological mechanisms across multiple scales ranging from molecular pathways to large-scale networks. At the micro-scale, signalling mechanisms, neuron to glia interactions, synaptic interactions and connectivity within local circuits and distributed network connectivity between different circuits can be studied in detail in animals and integrated with human data. At the meso-scale, ultra-high field magnetic resonance imag- ing (MRI) allows to investigate the intra-cortical, layer-specific structure and function of circuits even in human and non-human primates. At the macro-scale, wide-spread, inter-regional networks can be studied comprehensively, and their interactions can be computationally modelled. Novel positron- emission tomography (PET) tracers are available to quantify synaptic density in humans and animals in vivo. Moreover, brain networks contributing to specific cognitive readouts are understood in much more detail than just a decade ago thereby enabling to investigate the multi-scale underpinnings of neural resources within defined neurocognitive circuits. These advances pave the way to systematically investigate key defining properties of neural resources across multiple scales. One is their ability to accommodate increased cognitive demands through short and long-term plasticity. Another is to flexibly provide these plasticity-related benefits to different cognitive demands, a phenomenon referred to as transfer.
A major obstacle to understand how neural resources change over the lifespan in vivo has been that preclinical neurodegenerative and vascular pathology in seemingly healthy older adults remained hidden to scientists. Recent advances in biomarker assessment and PET imaging now allow for the first time to define physiological ageing in molecular terms by the absence of hidden and otherwise overlooked neurodegenerative pathology (e.g. amyloid and wide-spread tau). Furthermore, hidden vascular pathology can be visualised with ultra-high field MRI.
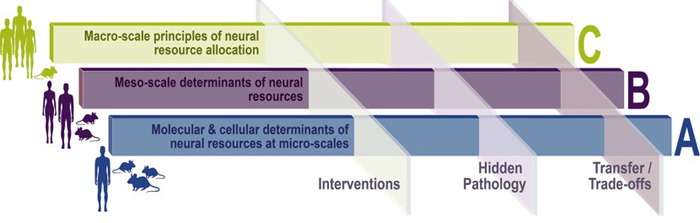
Magdeburg brings all these conceptual and technological advances together and is therefore in a unique position for achieving a multiscale characterisation of neural resources, their limitations and scope for increase. Our ultimate goal is to provide novel avenues for interventions to protect, support and increase neural resources over the lifespan encompassing and appreciating the complexity of the brain at different scales. To achieve this goal, we propose a CRC that is organised into three research areas covering micro- (research area A), meso- (research area B) and macro-scale (research area C) investigations in animals and humans. All research areas will be linked by inquiring into defined neu- rocognitive circuits, using interventions and plasticity-related transfer as a key feature of neural re- sources, taking into account hidden pathology and a common overarching conceptual framework. Three central projects will provide the necessary high-end technology and also the methodological foundation for micro- and meso-scale imaging, functional connectomics in animal imaging, and molec- ular PET imaging.
Our vision beyond the first funding period is that in twelve years we can individually determine:
- the scope for improving specific cognitive functions (e.g. memory, navigation or cognitive flexibility),
- the impact of preclinical pathology on specific brain circuits and the response of pathology to neural resource mobilising interventions (dissecting mechanisms of maintenance, resistance, resilience),
- the reserve mechanisms that are engaged and available,
- the neural, genetic and bodily, organ-overarching modifiers that are relevant.
On this basis, we envision to develop a comprehensive cognitive medicine framework to be able to individually tailor interventions to protect or enhance specific cognitive functions and optimise the transfer potential and minimise trade-offs of these interventions. From this personalised understanding of neural resources, we also envision to be able to predict and manipulate the specific cognitive trajec- tory of an individual.
Project groups and projects
for more information please click on the link below (A01-A08 / B01-B06 / C01-C05 / Z01-Z03)
| Code | Project title | PI |
| Molecular & cellular determinants of neural resources | ||
| A01 | The NMDA receptor complex - a signalling hub at the origin of cognitive flexibility? | Daniela C. Dieterich (Institute for Pharmacology & Toxicology, OVGU) Markus Fendt (Institute for Pharmacology & Toxicology, OVGU) |
| A02 | Shaping neuronal engram ensembles through excitation-transcription coupling | Anna Karpova (RG Neuroplasticity, LIN) Michael R. Kreutz (RG Neuroplasticity, LIN) |
| A03 | Neural resource allocation by spatial memory circuits facing progressive pathological challenges | Stefan Remy (Dept. Cellular Neuroscience, LIN) Stefan Dürschmid (Medical Faculty & University Clinic for Neurology, OVGU) |
| A04 | Cognitive enhancement by the anti- ageing protein Klotho – from molecular mechanisms to interventions | Emrah Düzel (Institute of Cognitive Neurology & Dementia Research, OVGU) Maria Andres-Alonso (RG Neuroplasticity, LIN) Michael R. Kreutz (RG Neuroplasticity, LIN) |
| A05 | Extracellular matrix integrity as neural resource of cognitive flexibility | Alexander Dityatev (AG Molecular Neuroplasticity, DZNE) Björn Schott (Univ. Clinic for Psychiatry & Psychotherapy, Georg-August University, Göttingen) Constanze I. Seidenbecher (Dept. Neurochemistry and Molecular Biology, LIN) |
| A06 | Neural resource mediated by BDNF- dependent neuroplasticity of cortico- hippocampal interactions | Volkmar Leßmann (Institute of Physiology, OVGU) Frank Ohl (Dept. Systems Physiology of Learning, LIN) |
| A07 | Orexinergic modulation of neural resource | Anne Albrecht (Institute for Biology, OVGU) Oliver Stork (Institute for Biology, OVGU) |
| A08 | The noradrenergic system´s contribution to neural resource in ageing | Matthew Betts (Institute of Cognitive Neurology & Dementia Research, OVGU) Dorothea Hämmerer (Institute of Cognitive Neurology & De- mentia Research, OVGU) |
| Meso-scale determinants of neural resources | ||
| B01 | Medial temporal lobe and prefrontal cortex connectivity as a neural resource for recognition memory | Matthias Prigge (RG Plasticity of Neuromodulatory Networks, LIN) Magdalena Sauvage (Dept. Functional Architecture of Memory, LIN) |
| B02 | Neural resources of mnemonic discrimination and their interaction with hidden pathology in older adults and SuperAgers | Radoslaw Martin Cichy (Dept. of Education and Psychology, Freie Universität Berlin) Emrah Düzel (Institute of Cognitive Neurology & Dementia Research, OVGU) |
| B03 | Grid cell integrity as a neural resource for navigation and episodic memory? | Kevin Allen (Univ. Hospital Heidelberg & DKFZ, Heidelberg) Thomas Wolbers (AG Aging & Cognition, DZNE) Jonathan Shine (Medical Faculty, OVGU) |
| B04 | Effects of hippocampal vascularization patterns on the neural resources of MTL neurocognitive circuits | Esther Kühn (Institute of Cognitive Neurology & Dementia Research, OVGU) Anne Maass (AG Multimodal Neuroimaging, DZNE) Stefanie Schreiber (Univ. Clinic for Neurology, OVGU) |
| B05 | Structural and functional determinants of attentional resources in multiple object and feature tracking | Jens-Max Hopf (Dept. Cognitive Neurophysiology, OVGU) Mircea Ariel Schönfeld (Dept. of Experimental Neurology, OVGU) |
| B06 | Mobilisation of neural resources for temporal attention | Eike Budinger (Dept. Systems Physiology of Learning, LIN) Toemme Noesselt (Institute for Psychology, OVGU) Janelle Pakan (Institute of Cognitive Neurology & Dementia Research, OVGU) |
| Macro-scale principles of neural resources and resource allocation | ||
| C01 | Dynamic modelling of training-induced, response-optimised mobilisation of neural resources | Marco Taubert (Institute of Sport Science, OVGU) Gabriel Ziegler (Institute of Cognitive Neurology & Dementia Research, OVGU) |
| C02 | Exploratory attentional resource allocation by the anterior prefrontal cortex | Max Happel (Dept. Systems Physiology of Learning, LIN) Stefan Pollmann (Institute for Psychology, OVGU) |
| C03 | Monitoring vs. Automatisation: Neural resource allocation for human skill learning | Elena Azañón (Medical Faculty & Univ. Clinic for Neurology, OVGU) Max-Philipp Stenner (Medical Faculty & Univ. Clinic for Neu- rology, OVGU)> |
| C04 | Restoring neural resources perturbed by sleep deprivation | Markus Ullsperger (Institute for Psychology, OVGU) |
| C05 | Intervening in circuits for cognitive resource allocation in primates | Kristine Krug (Institute for Biology, OVGU) Petra Ritter (Dept. of Neurology, Charité Berlin) |
| Scientific service projects | ||
| Z01 | Functional neural circuit analysis and small animal imaging in vivo | Frank Angenstein (AG Functional Neuroimaging, DZNE) Michael R. Kreutz (RG Neuroplasticity, LIN) Oliver Stork (Institute for Biology, OVGU) |
| Z02 | Human imaging at meso-scale | Esther Kühn (Institute of Cognitive Neurology & Dementia Research, OVGU) Oliver Speck (Institute for Physics, Dept. Biomedical Magnetic Resonance, OVGU) Michael Hanke (Institute of Systems Neuroscience, Heinrich Heine University Düsseldorf & Institute of Neuroscience and Medicine, Research Centre Jülich) |
| Z03 | Human molecular imaging ageing and SuperAgeing cohort | Emrah Düzel (Institute of Cognitive Neurology & Dementia Research, OVGU) Anne Maass (AG Multimodal Neuroimaging, DZNE) Michael C. Kreißl (Dept. of Radiology and Nuclear Medicine, OVGU) |
| Central project | ||
| IRTG | Integrated Research Training Group | Toemme Noesselt (Institute for Psychology, OVGU) Oliver Stork (Institute for Biology, OVGU) |
| Z | Central task of the CRC | Emrah Düzel (Institute of Cognitive Neurology & Dementia Research, OVGU) |






